Dynamics and mechanism of a light-driven chloride pump
Sandra Mous, Guillaume Gotthard, David Ehrenberg, Saumik Sen, Tobias Weiner, Philip J. M. Johnson, Daniel James, Karol Nass, Antonia Furrer, Demet Kekilli, Pikyee Ma, Steffen Brünle, Cecilia Maria Casadei, Isabelle Martiel, Florian Dworkowski, Dardan Gashi, Petr Skopintsev, Maximilian Wranik, Gregor Knopp, Ezequiel Panepucci, Valerie Panneels, Claudio Cirelli, Dmitry Ozerov, Gebhard F. X. Schertler, Meitian Wang, Chris Milne, Joerg Standfuss, Igor Schapiro, Joachim Heberle, Przemyslaw Nogly
Many biochemical processes in organisms - including our bodies as well as bacteria - are controlled by the transport of ions through membranes. For example, the function of our brain depends largely on ion transport: Ion gradients are involved in the generation of nerve signals. The controlled transport of ions is a fundamental process that requires the concerted interaction of different building blocks. Ion transport through membranes, so-called vectorial catalysis, is however not yet fully understood and cannot be artificially reproduced. Therefore, many research groups worldwide are interested in deciphering the function of different ion pumps.
Research Unit E in UniSysCat focuses on complex processes like vectorial catalysis. The aim is to understand the underlying concepts to pave the way for the controlled manipulation of catalytic systems in vivo. One possible application is, for instance, the development of novel optogenetic tools for neuroscience and cell biology.
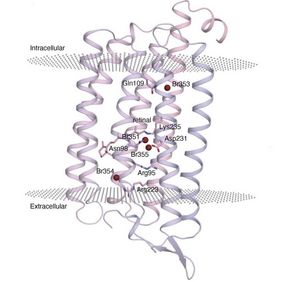
An international research team including the UniSysCat group of Prof. Joachim Heberle from Freie Universität Berlin was now able to decipher the detailed mechanism and the dynamics of a light-driven chloride pump relevant in optogenetics. The transport of chloride ions is a fundamental process in biology, regulating osmotic pressure, cell growth and membrane potential. The system studied is the chloride pumping rhodopsin of a marine bacterium, namely rhodopsin 3 from Nonlabens marinus (NmHR) that has already been employed as an optogenetic tool in the control of neuronal activity.
Bacterial rhodopsins like NmHR pump chloride ions from the extracellular medium into the cell. Like other rhodopsins, NmHR contains a light-sensitive component, the retinal. When the rhodopsin is illuminated, the retinal absorbs light and then changes its shape (isomerization), thereby initiating the ion transport process. Although bacterial rhodopsins have widely been studied and their structure is accurately known, the structural dynamics and the exact ion transport mechanism remain elusive.
Resolving the molecular mechanism of the ion transport in NmHR required a methodology with near-atomic spatial resolution and up to picosecond temporal resolution: the team combined time-resolved serial crystallography performed at a X-ray free electron laser facility, with time-resolved spectroscopy and multiscale simulations.
The time-resolved experiments enabled the researchers to generate a molecular movie of the chloride transport: In a first step, the ion uptake from the extracellular environment is supported by interactions with the retinal, and transport through a space generated by retinal isomerization occurs within about 1 microsecond of excitation. The release of chloride and the important blockage of backward flow is mediated by a salt bridge that forms an electrostatic gate at the intracellular face.
In addition, the research team could also describe in atomic detail how the light energy absorbed by NmHR is converted into kinetic energy driving the chloride transport. Interestingly, the photon energy absorbed by the retinal is stored in the form of charge separation between the isomerized retinal and its chloride counterion. Such a charge separation represents a fundamental feature for light energy conversion in nature as well as in technology.
The findings of Mous et al. have been published in Science: Mous et al., Science 375, 845–851 (2022), DOI: 10.1126/science.abj6663