Two recent UniSysCat publications show how protonation can control function in chemical and biological systems: The first work describes how the structural change of a bacterial photoreceptor is enabled by protonation. The second work shows that protonation of a catalyst is a crucial step to activate it for photocatalysis. These two examples demonstrate that protonation represents a coupling principle for processes that switch between states of different reactivity and functionality.
In the first publication, five UniSysCat research groups investigated local proton-linked electric field changes during the photoconversion of the dark state to the photoactivated state of a specific bacterial phytochrome called Agp2. The team involved the groups of Ingo Zebger, Maria Andrea Mroginski and Peter Hildebrandt from TU Berlin, Patrick Scheerer from Charité Berlin as well as Nediljko Budisa from University of Manitoba, Canada.
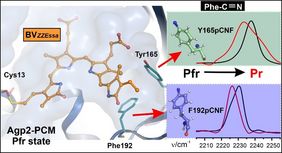
Phytochromes are light-sensitive proteins (photoreceptors) in plants, algae, bacteria and fungi. They control many light-dependent processes such as the greening of parts of plants or seed germination. All phytochromes contain a light-sensing tetrapyrrole chromophore which upon light-absorption causes the protein to switch from a physiologically inactive to an active form and back. These conversions involve chromophore and protein structural changes, which are coupled via the transfer of protons. Until now, the molecular mechanism underlying this structural change is not understood in detail. Following the hypothesis that local electric field changes are essential for coupling the motions of the chromophore and the protein, the research team presented a highly interdisciplinary study using a variety of methods from molecular biology, biochemistry, spectroscopy and theory. This integral approach allowed for determining electric fields sensed by nitrile reporter groups that were site-specifically introduced into Agp2. These groups offer the possibility to monitor local electric field changes during the photoconversion by probing the nitrile stretching frequency. Thus, it was shown that an intramolecular proton transfer step causes the largest field changes. These findings do not only provide deeper insights into the molecular functioning of phytochromes but contribute to identifying principles on how nature couples physical and chemical processes.
The impact of protonation steps on the reactivity also plays the crucial role in the second publication, which is dedicated to the conversion of the energy of sunlight into chemical, i.e. storable energy, an objective of utmost importance for a sustainable future energy system. This work, involving the groups of Peter Saalfrank, Roel van de Krol, Martin Oschatz, Reinhard Schomäcker, Shuang Li and Arne Thomas (together with Yan Lu from HZB) describes materials, which are composed out of abundant, non-toxic elements and can achieve hydrogen generation from water by photocatalysis.
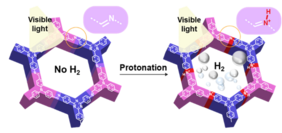
Covalent organic frameworks (COFs) are crystalline, porous, π-conjugated organic materials. As organic semiconductors they can act as photocatalysts for various reactions and are therefore thoroughly investigated in UniSysCat. The current work now sheds light on an important part of the photocatalytic mechanism: it is shown that protonation of the Schiff base moieties within the imine-linked frameworks is a crucial step to activate them for photocatalysis. Indeed, protonation yields a significantly improved light absorbance and charge separation efficiency, together with an increase in hydrophilicity of the COFs, which explains the largely improved photocatalytic performance. This work therefore contributes to a comprehensive understanding on the operation principles of organic photocatalysts and thus to a further optimization of these promising materials for sustainable energy solutions.
In essence, both publications describe the importance of a protonation-dependent control of the reactivity of the system. Whereas in phytochrome the intramolecular proton transfer enables the functionally relevant structural transition of a peptide segment, presumably via a drastic change of the local electric field. A strongly increased ability for photocatalytic hydrogen evolution in COFs is induced upon protonation, presumably as well via altering the local electrostatics. In a wider sense, changes of electrical fields play a crucial role in many light-dependent processes, e.g. when the energy of light is used to generate charge carriers, drive chemical reactions or enable structural changes. Protonation is probably the simplest process to control and tune electric fields in catalytic systems. The two publications show how such proton-linked electric field changes represent a coupling principle for processes that switch between states of different reactivity and functionality.
The first publication mentioned: Kraskov A, von Sass J, Nguyen DA, Hoang TO, Buhrke D, Katz S, Michael N, Kozuch J, Zebger I, Siebert F, Scheerer P, Mroginski MA, Budisa N, Hildebrandt P (2021). Local Electric Field Changes during the Photoconversion of the Bathy Phytochrome Agp2. Biochemistry, 60, 2967−2977. https://doi.org/10.1021/acs.biochem.1c00426
The second publication mentioned: J. Yang, A. Acharjya, M.-Y. Ye, J. Rabeah, S. Li, Z. Kochovski, S. Youk, J. Roeser, J. Grüneberg, C. Penschke, M. Schwarze, T. Wang, Y. Lu, R. van de Krol, M. Oschatz, R. Schomäcker, P. Saalfrank, A. Thomas, Protonated Imine-Linked Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution. Angew.Chem. Int. Ed. 2021, 60,19797–19803 https://onlinelibrary.wiley.com/doi/10.1002/anie.202104870