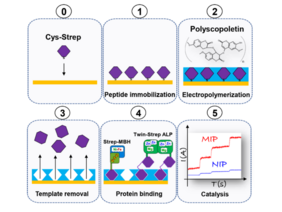
Understanding how nature harnesses enzymes to make valuable products is one of the major goals of UniSysCat. Biocatalysts are usually fragile molecules and their utilization requires incorporation of the enzymes in laboratory equipment such as an electrode surfaces or a solid carrier. Although various anchoring strategies are available, these procedures often apply to a narrow number of biocatalysts. In a current study, a team from five UniSysCat groups presents a novel approach to anchor enzymes on solid surfaces using the molecular imprinting strategy.
A biocompatible polymer based on the molecule scopoletin is manufactured with cavities able to selectively bind protein targets − similar to casting a mold. The idea behind this technique is to utilize the “lock and key” model that underlies substrate recognition by enzymes. This procedure requires the choice of a template molecule, which is imprinted into the scopoletin polymer. After its removal, the polymer contains cavities (called imprinting sites) that can be used to rebind the same molecule or compounds of interest that contain the template´s sequence. In order to provide a universal strategy for the immobilization of enzymes, the authors implemented the Strep-tag II affinity tag as template. This is a synthetic peptide, which is usually genetically included in recombinant protein sequences to allow the purification and detection of proteins by affinity chromatography. Using electrochemistry, surface-sensitive spectroscopy and molecular dynamics simulations the team gained an utmost control of the synthesis of the imprinted polymer. The functionality of this anchoring strategy was tested with two strep-tagged enzymes: an O2-tolerant [NiFe]-hydrogenase and an alkaline phosphatase. Both enzymes were successfully immobilized and retained their biocatalytic activities after multiple uses. The new method thus represents a simple, easy-to-use and potentially universal immobilization strategy with many advantages: It does not require polymer/protein functionalization, enables stable immobilization of enzymes and facilitates their sustainable use in biotechnological applications.
This study is an example of the fruitful interdisciplinary collaborations within UniSysCat and involves the expertise of the five UniSysCat groups of Oliver Lenz, Maria-Andrea Mroginski, Ulla Wollenberger, Frieder W. Scheller and Ingo Zebger. It has been published in Angewandte Chemie: Aysu Yarman et al., A Strep-tag Imprinted Polymer Platform for Heterogenous Bio(electro)catalysis, Angew. Chem. Int. Ed. 2024, e202408979. https://doi.org/10.1002/anie.202408979